Responding to Environmental Monitoring Results During a Cleanroom Garb Shortage
The Environmental Monitoring Trending Issue
When waves of COVID-19 cases first began crashing into hospital ICUs in early 2020, pharmacies quickly scaled up workflows in their cleanrooms to meet the surging demand for compounded drugs. Within weeks, pharmacies also began conserving cleanroom garb, to allow front line healthcare workers more access to PPE. As a result, cleanroom personnel spent more hours in less garb, preparing unprecedented amounts of Fentanyl, Midazolam, Norepinephrine, and other life-preserving medications.
To better understand the additional microbial toll that may have been placed on some cleanrooms, we spoke with one of our CAPS subject matter experts in environmental microbiology, Jamal Almustapha.

CAPS Sterile Compounding Review (CSCR): Hi Jamal. Early in the garbing shortage, some guidance documents suggested that pharmacies could limit the number of individuals working in the cleanroom to conserve garb. Was this always a practical approach?
Jamal Almustapha (JA): Limiting the number of personnel was a well-meant suggestion, but in the early stages of a national health crisis, it lacked practicality. Reducing cleanroom personnel at hospitals in places like New York City and New Jersey was not a viable pathway for meeting the sudden increases in demand for sterile compounded vasopressors, neuromuscular blocking agents, and other drugs needed to help keep patients alive.
CSCR: Then why would guidance suggest that a pharmacy limit its staff?
JA: Because the suggestion still makes sense from an objective point of view; if you have a limited number of cleanroom garb, then yes, limiting the number of people would conserve that garb. However, the guidance documents also included risk-based strategies for conserving garb when reducing staff was not possible.
To me, a practical solution to support patient care while ensuring environmental control in the cleanroom was to implement limited cleanroom garbing in the room, while maintaining sterile gloves and sterile sleeves inside the ISO Class 5 hoods, because those are the critical areas where direct compounding occurs.
CSCR: If personnel must wear less garb, isn’t there always potential impact on the environment?
JA: Absolutely. People are always a common source of contamination in a cleanroom. People shed the most particles, contaminate the most surface areas, and are always the most general variable when it comes to microbial activity inside a controlled space. Having a comprehensive garbing program prevents people from naturally contaminating their surrounding environment which ensures an appropriate level of environmental assurance in the cleanroom.
With that in mind, it’s logical to assume that utilizing less garb will consequently elevate the viable and non-viable particulates in a cleanroom area. A risk-based garbing conservation strategy should protect the most vulnerable area― the ISO Class 5 workstation where sterile drugs are transferred into their final containers.
CSCR: What can undermine a risk-based garbing conservation strategy?
JA: Unfortunately, there’s no “one size approach” that will work for every sterile compounding program. Workflows, occupancy levels, and airflow designs are just some of the variables that will influence the success or failure of your garbing conservation strategy.
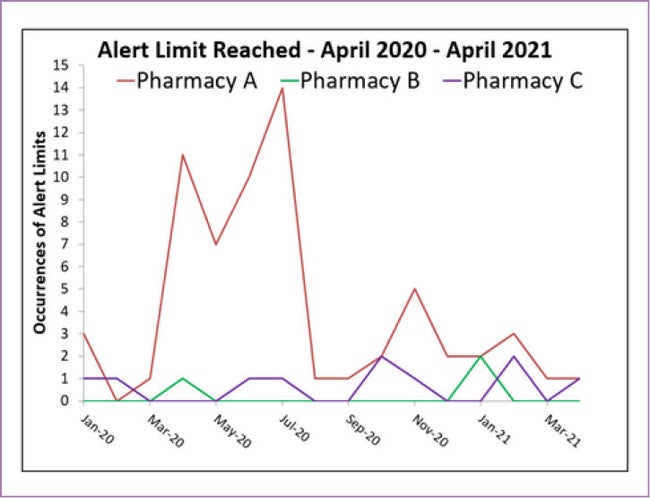
CSCR: That’s so true, Jamal. When I compare environmental monitoring results from pharmacies that we consulted with during last year’s garbing shortages, some cleanrooms clearly depended more on the garbing policies than others (see figure 1). It’s as though eliminating a garbing requirement could expose some other pre-existing weakness that was always waiting to reveal itself.
JA: One weakness may be cleanroom behavior from individuals. People should always suspect that their actions may elevate the particulate activity. Therefore, they should also elevate their aseptic behavior. No matter what the situation, everyone must make a conscious decision to be mindful of their movements throughout all the activities within the cleanroom.
CSCR: How do pharmacies decide when their garbing conservation isn’t working?
JA: Their most valuable asset to help make these types of decisions is a robust Environmental Monitoring (EM) program.
CSCR: What does it mean to have a “robust” Environmental Monitoring Program?
JA: Instead of only taking samples sporadically, a robust EM program utilizes frequent sampling for viable microbial isolates and then uses that data to establish trends.
One set of sample results will only provide a “snapshot” of the microbial activity at one point in time. Whereas frequent viable sampling, with subsequent trending of the quantity and identification of the microbial isolates, can better alert pharmacies when process changes or personnel have impacted environmental control.
CSCR: Can you further explain the disadvantages of using a “snapshot” approach?
JA: Remember, a viable result isn’t even current information. After the proper incubation time is accounted for, it represents conditions from days ago. More importantly, if you only take viable air samples every six (6) months per minimum USP standards, you really only capture microbial conditions for two days out of the year. A lot may get missed with twice-a-year sampling.
Also, by having such an elongated time from one sample to the next, there is less to be learned about an actionable result. How long was an adverse condition present? What was the root cause? Critical answers become very unlikely.
CSCR: Can you give us any advice to make better decisions during a garbing shortage?
Wherever possible, use objective data to make your decisions, not assumptions about the cleanroom. The most important advice I can give is to continuously do whatever is necessary to protect those in need of your help. And when it’s time to make a tough call, always take the side of your patient.
Recap
In a past newsletter article, we discussed how tracking and trending room certification data could help prevent equipment failures in the cleanroom. Using a similar rationale, one can also trend environmental monitoring data to prevent actionable microbial excursions. Microbial excursions, however, are primarily caused by humans, who can be less predictable, and a lot more mobile, than a stationary piece of equipment.
Therefore, the strength of your environmental monitoring program will depend on frequent sampling and effective trending.
Nobody enters a cleanroom intending to contaminate. Perhaps learning a new process, however, or struggling to keep up with unprecedented demand, can distract individuals from very important aseptic principles. Is your team always ready for the never-ending changes? Microbial trending can help you to continuously answer that question.
Figure 1
Comparison of alert limit frequency at three pharmacies, all conserving cleanroom garb.
Compared to pharmacy B and C, pharmacy A noted a much larger spike.
By using frequent sampling, trending, and alert limits, pharmacy A was able to adjust their practices and reduce the alert limits for the remainder of the year.
Stay Ahead of the Curve

Enhancing operational, quality, and standardization initiatives in the hospital pharmacy is within your reach. Through CAPS Consulting, our team of pharmacy experts can help bring your sterile compounding program to the next level.
Elevate Your Sterile Compounding Program
The first step is simple. Connect with a sterile compounding consultant and find out how our programs can be customized to meet your needs.